
Smart Wireless Implant for Infection Monitoring SWi2M
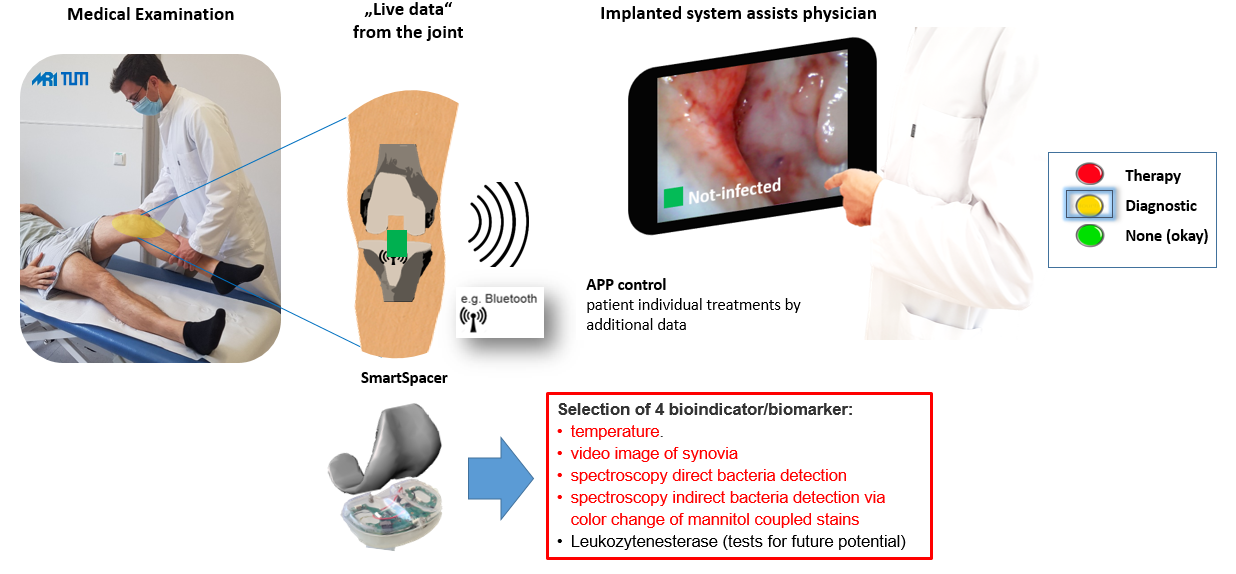
Schematic representation of the clinical workflow
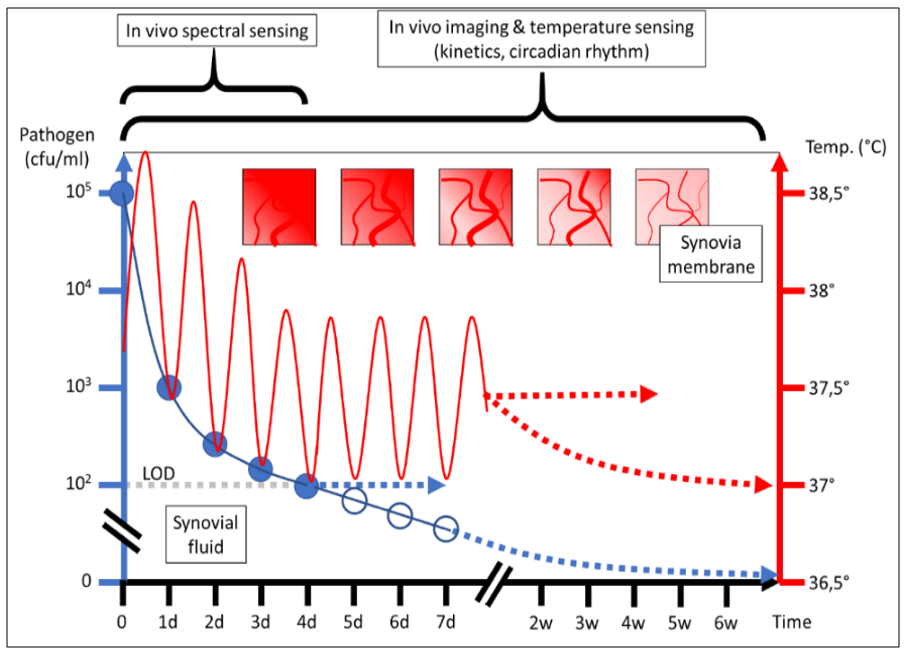
Graphical illustration of multiparametric data acquisition
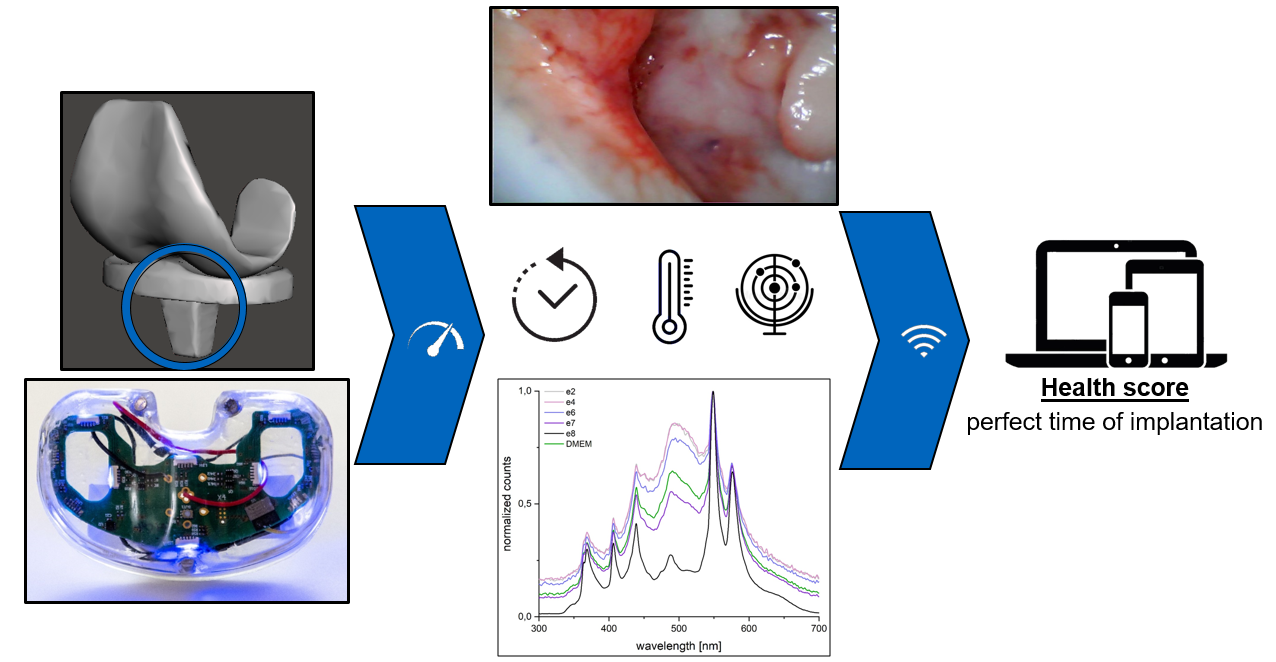
Principle of data acquisition and transmission
Primary total knee arthroplasty (TKA) is an extremely successful treatment procedure with excellent clinical outcomes. However, serious periprosthetic joint infections (PPI) occur in ~2% of patients after primary TKA and in ~15% of patients after revision total knee arthroplasty (RTKA). Thus, PPI is the most common cause of early TKA failure in the United States. Despite advances, not only is the absolute number of prosthetic infections increasing, but the relative risk of infection is also increasing due to increasing comorbidities, aging, and increase in antimicrobial resistance [1]. The economic burden associated with PPI is enormous. The cost of PPI treatment in 2020 is estimated at $1.6 billion for the US market alone [2].
Low-grade infections present a special challenge. The diagnosis of low-grade PPI is complex and time-consuming, since it is "only" an underlying infection that leads to nonspecific symptoms such as pain, restricted mobility, and a high degree of suffering on the part of the patient, but not to strong systemic signs of infection such as fever or fatigue. To make the complex diagnosis of low-grade PPI, several clinical criteria must be included: (1) systemic signs of inflammation, (2) analyses of the synovial fluid of the affected knee joint, (3) microbiological and (4) histopathological evaluations. In therapy, two-stage revision including the use of a temporary space holder (spacer) is still considered the gold standard for revision in low-grade PPI nowadays. Especially in cases with unidentified or difficult-to-treat multidrug-resistant microorganisms or compromised soft tissue conditions. The procedure for revision surgery is shown schematically in Figure 1. In the first operation, the inserted KTEP is explanted and a spacer made of antibiotic-containing polymethyl methacrylate (PMMA) is inserted, such as the gentamicin-containing bone cement Palacos® from Heraeus Medical. In addition, systemic antibiotic therapy is administered. After a time interval of 2 to 6 weeks, the spacer is surgically removed and a final revision knee endoprosthesis is inserted.
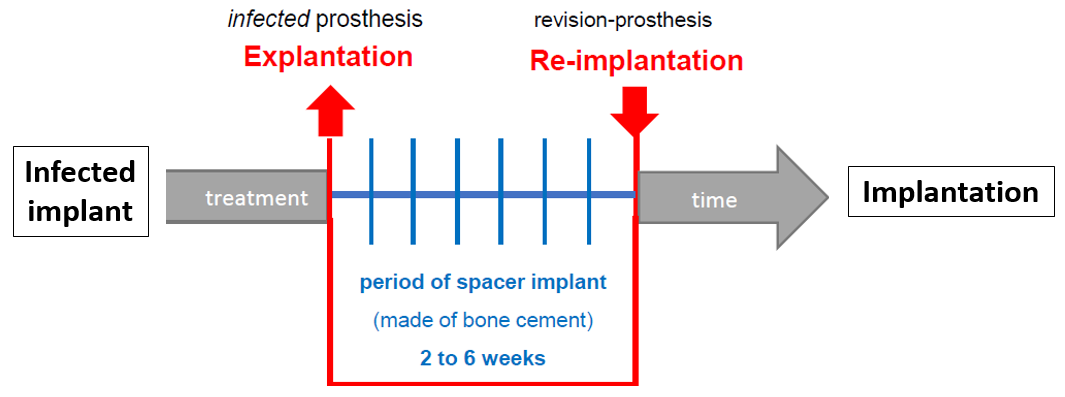
Spacerinterval
However, there is no evidence to day regarding the necessary length of the time interval between the two surgeries [3, 4]. Parameters currently used as evidence of healing of an infected knee joint are based " only" on (1) a decrease in swelling and hyperthermia and (2) normalization of C-reactive protein (CRP) levels in the blood as a surrogate marker for inflammation [5]. However, these criteria have low evidence in clinical trials because swelling and hyperthermia are only subjectively assessed by the physician [4] and slow CRP kinetics may be influenced by the patient's general health (including influenza, urinary tract infection, dental inflammation, etc.) [6]. Moreover, no reliable correlation was found between persistent infection and serum markers. Invasive diagnostics such as multiple punctures or arthroscopies have also been shown to be ineffective in ruling out persistent infection [4]. Therefore, no diagnostic clinical tool currently exists for continuous monitoring or detection of infection eradication.
The aim of the SWi2M project is therefore to develop a novel digitized SmartSpacer for precise longitudinal "4D" tracking of infection in two-stage KTEP replacement that can support personalized therapy decisions. The project will develop a knee spacer with integrated sensors for in vivo diagnostics and evaluate it in live studies. This novel digitized SmartSpacer will provide continuous sensory and imaging data telemedically for the treating physician to assess the disease process. Due to the spacer design space, sensors, power sources and readout electronics can be embedded in PMMA. In summary, the SmartSpacer is expected to provide the following benefits.
- continuous monitoring of infection eradication and personalization of the ideal time for reimplantation of the final KTEP.
- minimization of postoperative complications including persistence of infection
- analysis of longitudinal in vivo data
- telemedicine application in ambulatory patients
- cost reduction for the hospital and the health care system due to tailored, patient-individualized therapies
- accelerated recovery of independence with mobility for affected patients due to individualized revision timing
References
1. Lee, D.-H., et al., Causes and Clinical Outcomes of Revision Total Knee Arthroplasty. Knee surgery & related research, 2017. 29(2): p. 104-109.
2. Palmer, J.R., et al., The treatment of periprosthetic joint infection: safety and efficacy of two stage versus one stage exchange arthroplasty. Expert Rev Med Devices, 2020. 17(3): p. 245-252.
3. Schwarz, E.M., et al., 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. Journal of Orthopaedic Research, 2019. 37(5): p. 997-1006.
4. Muhlhofer, H.M.L., et al., Synovial aspiration and serological testing in two-stage revision arthroplasty for prosthetic joint infection: evaluation before reconstruction with a mean follow-up of twenty seven months. Int Orthop, 2018. 42(2): p. 265-271.
5. Eisenhart-Rothe, R.v. and R. Burgkart, Estimates of case numbers for TEP revisions in Germany for health economic evaluation of smart joint spacer. 2019, Clinics for Orthopaedics and Sportorthopaedics, Technical University Munich: München.
6. Grimberg, A., et al., Endoprothesenregister Deutschland - Jahresbericht 2019, in EPRD-Jahresbericht, A. Grimberg, et al., Editors. 2019, Deutsche Gesellschaft für Orthopädie und Orthopädische Chirurgie (DGOOC).
